Peptoid Self-Assembly
Page Content being updated September 2025
Page under construction…
Introduction
Molecular components in nature are spatially organized and compartmentalized to generate function. Convenient and versatile synthetic material platforms to perform analogous functions are lacking. The Lau Lab specializes in synthetically convenient peptoids, surface functionalization, and nanopores arrayed in nanoporous alumina membranes to fill this niche.
Peptoids are synthetically convenient, sequence-specific polymers (see Peptoids page), and are ideally suited for constructing molecular scaffolds with unit spacing corresponding to the single peptoid residue (0.34 nm). Peptoid sequences may therefore be used to arrange molecular functionality on a scale too small for nanolithography and much longer than conventional synthetic molecules (on the order of 1~10 nm). In particular sequences consisting of 5 or less residues are of great interest for self-assembly. The low number of residues allows for an easier time balancing conditions, such as solvents, as well as an easier time scaling.
FF Peptoids
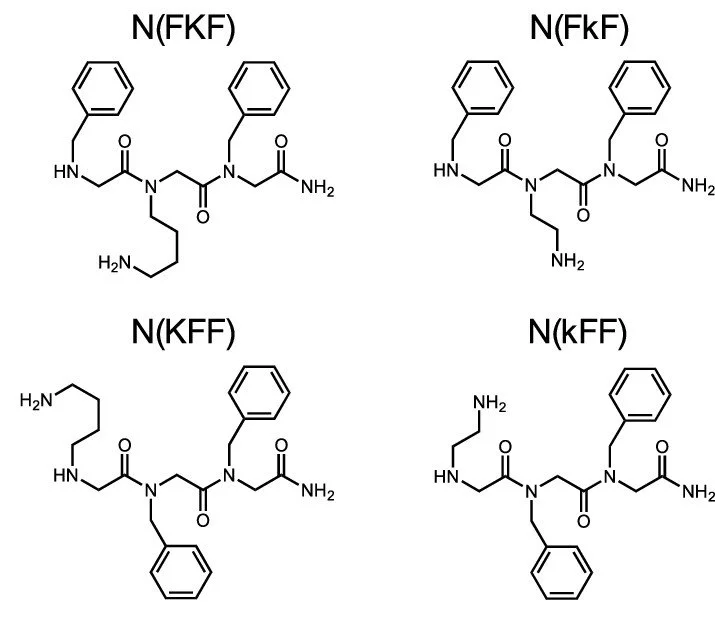
As Peptoids are peptide mimics, discoveries can start at the peptide isomer. Such is the case for diphenylalanine (FF). This dipeptide was found to be the key aggregating domain of amyloid peptides. FF and its derivatives inspired aqueous self-assembling peptoids that can function without directing influence of other components. Four achiral peptoid trimers arose from research testing the minimal requirements for nanostructure assembly of peptoids/peptides that are directly soluble in water. Every sequence contains 2 residues of N-benzylglycine (Nf), the analog of phenylalanine (Phe), and either N-(4-aminobutyl)glycine (Nk) or N-(2-aminoethyol)glycine) (Nke) in either the 1st or 2nd position in the sequence. (see image to the right.)
The peptoids from left to right, top to bottom can be written as NfNkNf, NfNkeNf, NkNfNf, NkeNfNf. Current naming emphasizes the peptide basis.
Aromatic and Aliphatic structures
The aromatic structure of the F peptide and the Nf peptoid allows for an obvious directionality for pi-pi stacking and dispersion interactions. This self-assembly is strong, and stable, but difficult to reverse. This presents challenges in designing dynamic systems and in managing material life-cycles. This brings the motivation to move away from the usage of aromatic structures in self assembly. Our research had yielded several novel surface-active and emulsion stabilizing tetrapeptides containing the aliphatic amino acids leucine (L), isoleucine (I) and valine (V). Aliphatic side chains are associated with self-assembly mechanisms similar to amyloid core sequences from degenerative disease proteins. Further, β-sheet secondary structures can be promoted in the order L/V > A > G.
Interfacial Crystallization
In minimalist peptide and peptoid assembly there are common triggers to control assembly and crystallization, such as temperature and pH changes. Modification of solvation has received less attention. We have however shown that an acetylated FF peptoid analogue may form stacked nanosheet plates or crystalline needles depending on whether water is used to induce precipitation from an acetonitrile stock solution (i.e., kinetic assembly) or DMSO is added to a water based solution to slow evaporation (i.e., thermodynamic assembly). Many studies highlight the importance of taking a more holistic solvent plus solute view of assembly.
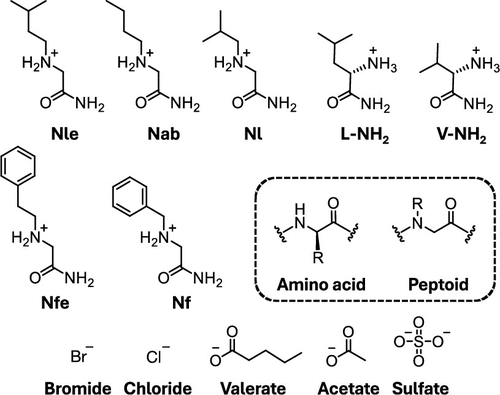
Air–liquid, liquid–liquid, and liquid–solid phase boundaries arise in many chemical contexts. These interfaces themselves may direct the formation of structures not otherwise accessible and permit additional control over material functionality. The aliphatic side chains mentioned previously form an important role in the formation and stabilization of interface-spanning crystalline sheets at liquid interfaces in a process we term interfacial crystallization (IFC). We first observed IFC during recrystallization of halide salts of a set of peptoid monomer amides with aliphatic and aromatic side chains. The interfacial sheets were observed to grow and fill entire cross sections of solution containers. We studied different peptoids and anions(See image to the right), and compared the results with key amino acid analogs (i.e., amino acid amides). The results provided generalizable molecular design insights for IFC and potential mechanistic explanations for this growth process.
shown to the left here is an air-liquid interface IFC.
Below is shown Peptoid and Amino Acid Amides and Counterions Studied.
Nanostructures
As peptoids are peptide mimics they fold and create structures based on interactions between different sections of the chain. These secondary structures, helices, ribbons and sheets, can form into tertiary structures. Nanosheets are the most common structures, with strips of these being able to curl into nano- and microtubules. Research has also given rise to nanospheres, mimicing spherical peptide micelles.
Nanopores
Nanopores are simply defined by their diameters and lengths but their straightforward geometry may already be used to organize molecular components for advanced function. A prime example is the nuclear pore complex (NPC). NPCs’ multiple 50~100 nm ring-like nucleoporin protein scaffolds, lined with natively unfolded polypeptide sequences, regulate all nucleocytoplasmic protein and RNA transport. Inspired by these biological pores, the Lau Lab functionalize nanoporous alumina membranes, produced by an inexpensive self-organized anodization process, with soft matter to control the surface chemistry of these nanopores and consequently the diffusion of macromolecules through them. These artificially gated nanopores will enable chemically specific molecular separation and enhance our mechanistic understanding of NPCs.
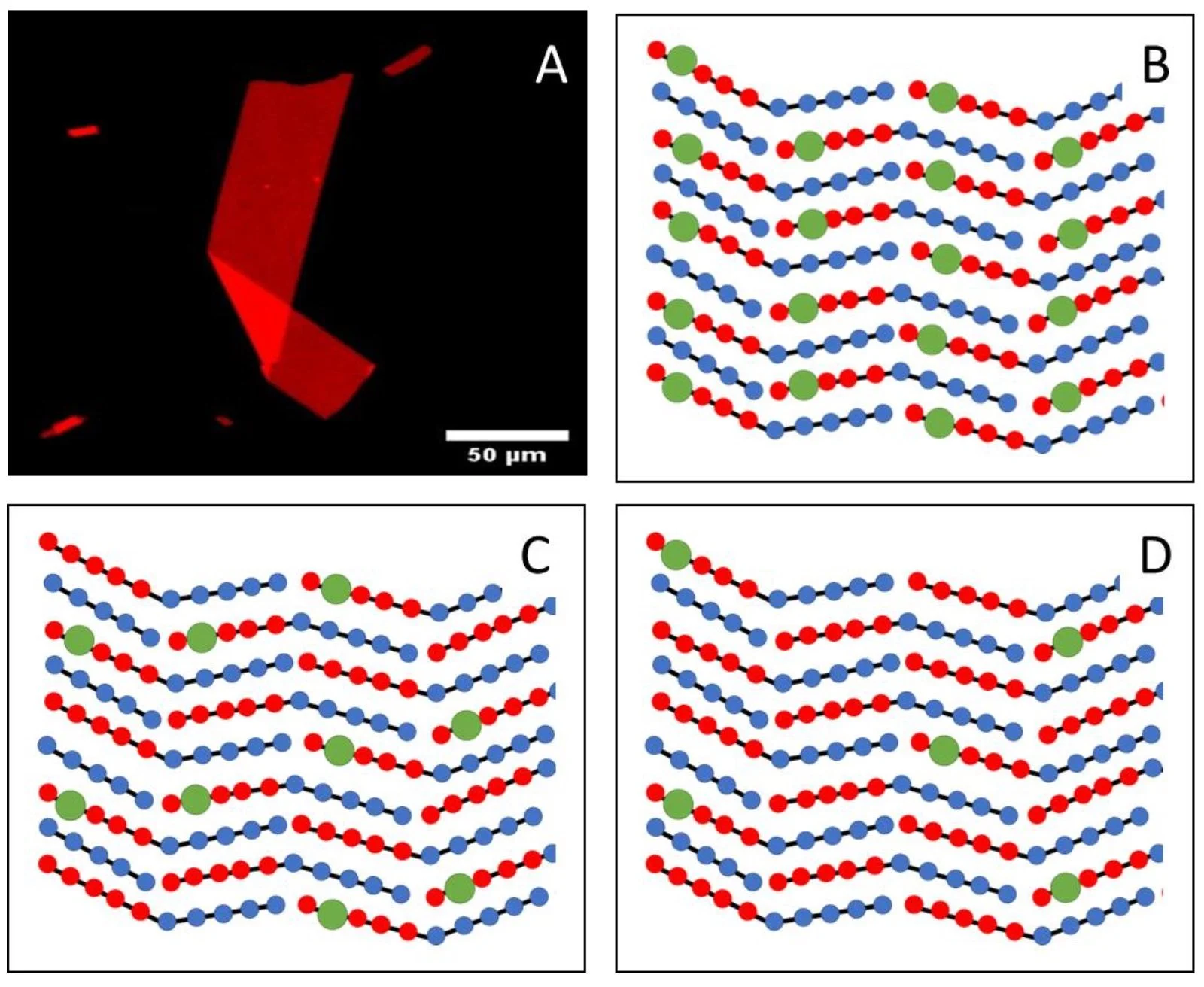
Peptoid Nanosheet as a functional scaffold (Nile Red stained) — green dots in schematics indicate coupling sites possible by controlling assembly design.
Spatial definition of chemical groups with peptoids